Oral, Brain-Penetrant MasterKey Inhibitor of Oncogenic EGFR Mutations
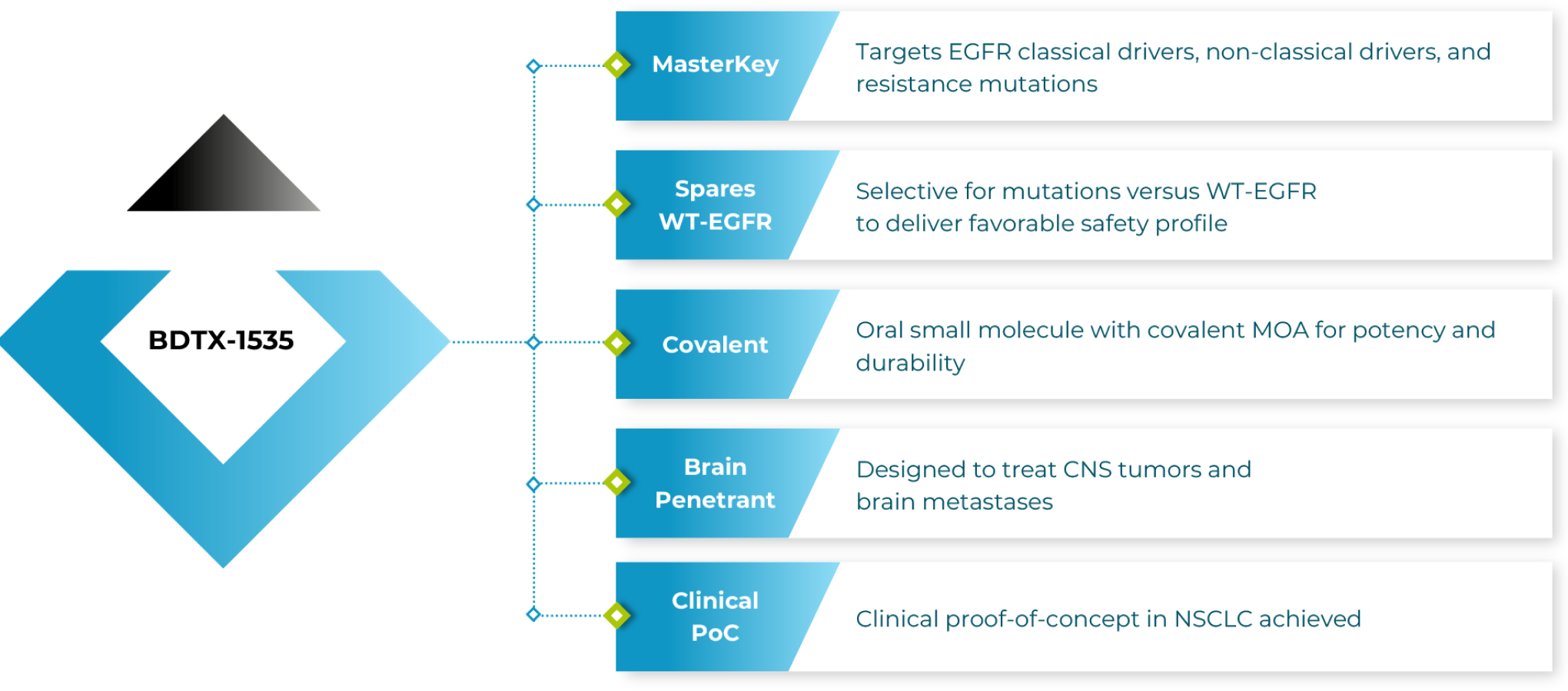
NSCLC accounts for approximately 85% of lung cancer cases worldwide. Up to 20% of patients with NSCLC in North America and Europe and up to 50% of those in Asia harbor mutations in EGFR. BDTX-1535 was specifically designed to address the broad spectrum of EGFR mutations that can drive NSCLC.
- Classical driver mutations: L858R and Ex19del
- Non-classical driver mutations: found in 20-30% of patients
- Acquired resistance mutations: C797S
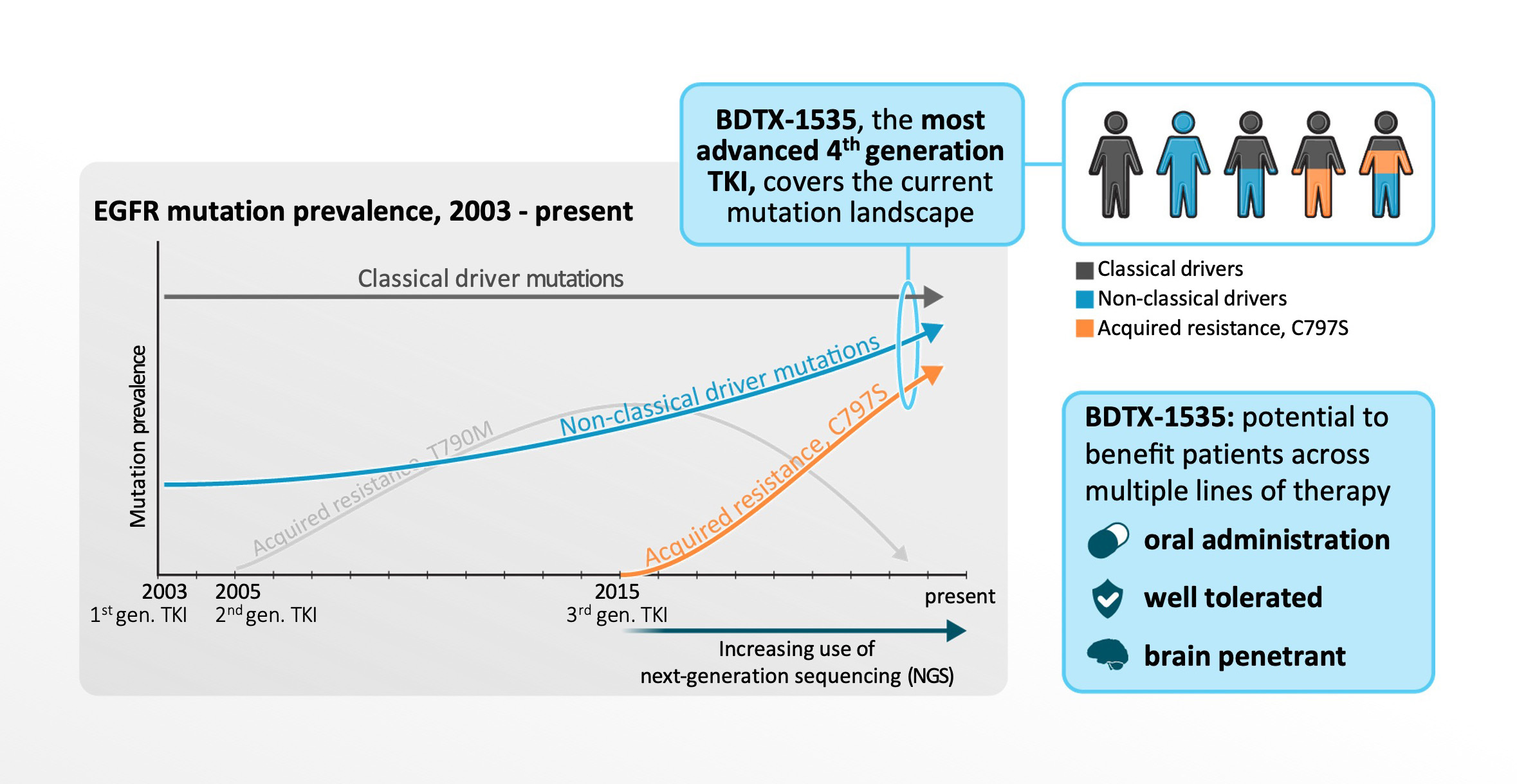
The real-world spectrum of EGFR mutations has changed with the increased use of NGS and the evolving treatment landscape. BDTX-1535 is uniquely positioned as a first and best-in-class 4th-generation EGFR inhibitor that could potentially benefit both 1st-line and 2nd-line patients with NSCLC.
BDTX-1535 demonstrated clinical proof-of-concept in a Phase 1 trial and data were presented in 2023. BDTX-1535 is currently in a Phase 2 trial for patients with either 2nd/3rd-line or 1st-line NSCLC. Learn more at clinicaltrials.gov.